Scientists around the world are racing to develop and test a Covid-19 vaccine.
Whichever vaccine passes regulatory hurdles first is set to be the fastest ever vaccine developed. But does this speed mean that safety will be compromised?
Health watchdogs, such as the UK's Medicines and Healthcare Products Regulatory Agency (MHRA) have said there is “absolutely no chance”.
Here are your questions answered about the history of vaccine development and safety.
Why are scientists working at such speed?
The new coronavirus has spread to almost every corner of the planet – more than 51 million people have been infected which has led to more than 1.2 million deaths.
But the Covid-19 pandemic has not only had a devastating impact on human health, it has also severely impacted global economies.
Gordon Dougan, professor of vaccine medicine at Cambridge University, said: “I think the reason vaccines are being developed so quickly is the urgency of the situation.
“We’ve got a global pandemic and there’s a need to respond and so the vaccine community has really moved very, very quickly. And the reason it’s moving so quickly, is the health crisis, and also the economic crises caused by the pandemic.”
How are vaccines being developed so quickly?
Prof Dougan added: “The actual disease itself, and the antigen which is used, or the piece of the virus that’s used for protection, is well definable. So that allows [scientists] to get off to a rapid start to set the normal processes in motion.”
He said there was an “unprecedented field of candidates," with nearly 200 vaccine candidates in trials.
Dr Claire Waddington, clinical lecturer in infectious disease at Cambridge University, said the speed is down in part to our modern ability to do things like genome sequencing.
“Some of it is reflecting modern science in that, certainly for the Oxford vaccine, they basically had a vaccine, almost ready to go where you could just plug in the genetic code. But the background to the vaccine was already established and being used for other things," she said.
“And that’s in vast contrast to when vaccines were developed 40, 50 years ago where essentially we didn’t have any of that genomic knowledge or the sort of scientific capability to do such specific planning."
Will it be the quickest vaccine ever developed?
There have also been other illnesses – such as Ebola – which have seen rapid vaccine development.
But experts believe that this could be the speediest ever.
Dr Waddington added: “It’s usually in a realm of about 10 years to develop a vaccine. I think the record is around, maybe three or five years.”
Will safety be compromised?
England’s deputy chief medical officer Jonathan Van-Tam said the standards are “no lower” because this is a public health emergency.
In a Downing Street data briefing, Professor Van-Tam set out how the timetable for developing and approving a vaccine had been condensed from the usual number of years due to the coronavirus crisis.
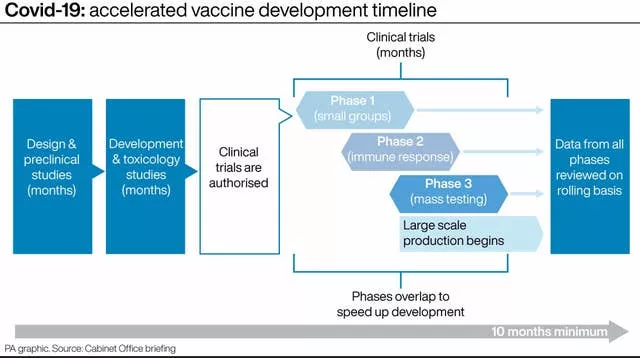
He said the three phases of clinical trials overlapped, instead of taking place sequentially, and pharmaceutical firms had begun manufacturing before final approval had been granted – taking on the risk that they may be forced to scrap their work.
Prof Van-Tam said said that the three phases of clinical trials were no smaller and “the
standards are no lower just because this is a public health emergency”.
What does that mean for regulation?
The new way of working means that regulators around the world can start to look at scientific data earlier than they traditionally would do.
Prof Van-Tam described how data from each phase of the clinical trial can be put to regulators in what has been called a “rolling review” – instead of regulators going through reams of information at the conclusion of the trials.
Dr June Raine, chief executive of the MHRA, said: “A Covid-19 vaccine will only be approved once it has met robust standards of effectiveness, safety and quality.”
“Our teams of scientists and clinicians carefully, methodically, scientifically, rigorously review all the data on safety, effectiveness and quality.
“The public can be confident that all those tests are done to the very highest standards. Safety is our watchword.”
How do you monitor safety for new medicines?
Prof Dougan said: “When you administer the vaccine for the first time, you often get signals very early on – so you know yourself when you’ve had a vaccine sometimes because you get a little reaction to it.
“And so a lot of the events to do with safety are very early events and they’re sort of obvious.
“Then you have these things what we call adverse events that are actually rarer. So as you increase the number of people who you vaccinate you will then identify individuals in the population that will have an ‘unusual setup’ either because of the clinical condition or the genetic setup, and these are often what we call rare events but you’ll pick them up as you go through the process towards phase three (clinical trials).
“And then what you would normally do is you would also monitor a vaccine after licencing. And then look for the even rarer events or any longer term events that you might see.”