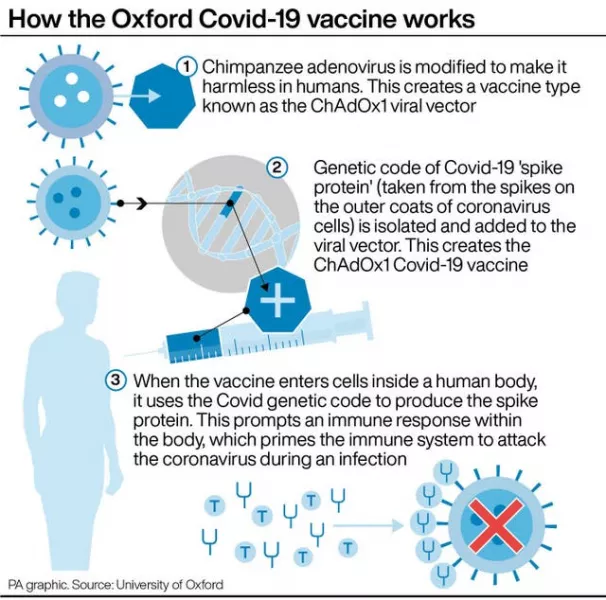
As the European Union’s medicines regulator prepared to consider clearing AstraZeneca’s Covid-19 vaccine for use, a draft recommendation from a German expert committee called for offering it only to under-65s for now — adding to the spotlight on the vaccine.
And amid a heated dispute between the EU and the Anglo-Swedish company over its plans to deliver doses to the 27-nation bloc, the EU’s executive commission asked Belgian authorities to inspect a factory in Belgium that is part of the vaccine’s European production chain.
The European Medicines Agency (EMA) is expected to clear the AstraZeneca vaccine for use in the EU on Friday. It would be the third approved in the EU, after the BioNTech-Pfizer and Moderna vaccines.
European authorities are under pressure after a sluggish start to the EU’s vaccination campaign in its first month, and the AstraZeneca vaccine would add much-needed extra supplies.
However, Brussels and the drugmaker are at loggerheads over expected delays in deliveries.

AstraZeneca said last week that it planned to cut initial deliveries in the EU from the scheduled 80 million doses for the first quarter of the year to 31 million doses because of reduced yields from its manufacturing plants in Europe, but the EU suspects doses produced in Europe have been directed elsewhere.
And the German draft recommendation adds a question mark over how widely it might be used — though the country’s health minister stressed that a final decision will not be made until after Friday’s EMA meeting.
Germany’s vaccination advisory committee, an independent panel advising the government, called for using AstraZeneca’s vaccine for the 18-64 age group on the basis of currently available information.
It said that “there currently is not sufficient data to assess the vaccination effectiveness from 65 years”.
AstraZeneca said after Thursday’s release of the German draft that “the latest analyses of clinical trial data for the AstraZeneca/Oxford Covid-19 vaccine support efficacy in the over 65 years age group”. It added that it awaits EMA’s decision.
The company noted earlier this week that British regulators supported its use in the older age group despite lack of late-stage effectiveness data.
It pointed to earlier-stage data published in the journal Lancet in November “demonstrating that older adults showed strong immune responses to the vaccine, with 100% of older adults generating spike-specific antibodies after the second dose”.
But questions have remained about how well the vaccine protects older people.
Only 12% of participants in the AstraZeneca research were over 55 and they were enrolled later, so there has not been enough time to see whether they get sick at a lower rate than those who did not get the vaccine.
German Health Minister Jens Spahn said there had been a discussion about a lack of data on the subject since the autumn, but it was not yet clear “how concretely” that would ultimately affect authorities’ decisions.
AstraZeneca said on Thursday that the latest analyses of clinical trial data “support efficacy in the over 65 years age group” and it awaits the EMA’s decision.
The EU, which has 450 million people, has signed deals for six different vaccines.
In total, it has ordered up to 400 million doses of the AstraZeneca vaccine and sealed deals with other companies for more than two billion shots.
The European Commission asked the Belgian government to inspect manufacturer Novasep’s factory in Seneffe, Belgium due to dissatisfaction with AstraZeneca’s explanations for its inability to deliver all the EU’s expected doses on time.
However, the Commission said it remains confident that the AstraZeneca delay will not affect its plans to ensure that at least 80% of EU citizens over age 80 are vaccinated by March.
Constructive tone in our exchange with @AstraZeneca CEO Pascal Soriot, in our Vaccine Steering Board, on deliveries of their vaccine following approval. The EU remains united & firm ➡️ Contractual obligations must be met, vaccines must be delivered to EU citizens.
— Stella Kyriakides (@SKyriakidesEU) January 27, 2021
Health policy spokesman Stefan de Keersmaecker said that target is based on the availability of doses manufactured by Pfizer-BioNTech and Moderna.
“It is an ambitious target, but we believe it is a realistic one,” he said.
A third round of talks on Wednesday between AstraZeneca and EU officials produced no immediate results but the Commission said it was hopeful of a resolution.
More than 400,000 EU residents with Covid-19 have died since the beginning of the pandemic.
According to the EU, the Belgian factory is one of four AstraZeneca sites included in the contract sealed by the Commission and the company to produce vaccines for the EU market.
Stella Kyriakides, the European Commissioner for health and food safety, said AstraZeneca should provide vaccines from its UK facilities if it is unable to meet commitments from factories in the EU.
Company chief executive Pascal Soriot has argued in an interview with Die Welt newspaper this week that the UK government helped develop the vaccine and signed its contract three months before the EU reached its agreement.
He said the contract with British authorities specifies that vaccines produced at UK sites must go to the UK first.
Mr Soriot said that “after we have reached a sufficient number of vaccinations in the UK” the company will be able to use the British sites to supply Europe as well.
Ms Kyriakides also made clear the EU would find out if some of the doses manufactured in the EU were diverted elsewhere.