The world’s first study which will deliberately expose volunteers to coronavirus to establish the smallest amount of virus needed to cause infection will take place in the UK.
hVIVO, a subsidiary of Dublin-based Open Orphan, is an industry-leading clinical company that will carry out the Covid-19 “human challenge” research backed by the UK government.
Starting in the next few weeks, the trial will involve up to 90 carefully selected, healthy adult volunteers being deliberately exposed to Covid-19 in a safe and controlled environment.
The first-of-its-kind study has been approved by the UK’s clinical trials ethics body.
It will give doctors a greater understanding of Covid-19 and help support the pandemic response by aiding vaccine and treatment development.
Because the safety of volunteers is paramount, this virus characterisation study will initially use the version of the virus that has been circulating in the UK and Ireland since March 2020.
This variant has been shown to be of low risk in young healthy adults.
Medics and scientists will closely monitor the effect of the virus on volunteers and will be on hand to look after them 24 hours a day.
The researchers are working closely with the Royal Free Hospital and the North Central London (NCL) Adult Critical Care Network to ensure the study will not affect the British health service’s ability to care for patients during the pandemic.
The study will not begin without their go-ahead, the UK's Department for Business, Energy and Industrial Strategy (Beis) has announced.
Important role
hVIVO’s chief scientific officer Dr Andrew Catchpole, said Covid-19 “human challenge” studies have the potential to play an important role in providing data and information that will help continue to develop vaccines to control the pandemic.
Dr Catchpole said the challenge model can answer “a wide range of fundamental scientific questions that are not feasible with traditional field trials, such as exactly what type of immunological response is required to confer protection from re-infection.”
Interim chairman of the UK Vaccines Taskforce, Clive Dix, said: “We have secured a number of safe and effective vaccines for the UK, but it is essential that we continue to develop new vaccines and treatments for Covid-19.
“We expect these studies to offer unique insights into how the virus works and help us understand which promising vaccines offer the best chance of preventing the infection.”
After the initial study has taken place, vaccine candidates proven to be safe in clinical trials could be given to small numbers of volunteers who are then exposed to coronavirus.
This will help identify the most effective vaccines and accelerate their development.
People aged between 18 and 30 years old, who are at the lowest risk of complications resulting from coronavirus, are being encouraged to volunteer for this vital study.
Volunteers will be compensated for the time they spend in the study.
Accelerate new treatments
In the past, human challenge studies have played important roles in accelerating the development of treatments for diseases including malaria, typhoid, cholera, norovirus and flu.
The trials have also helped researchers establish which possible vaccine is most likely to succeed in phase three clinical trials that would follow, usually involving thousands of volunteers.
This study, backed by a £33.6 million (€38.6 million) UK government investment, will also help doctors understand how the immune system reacts to coronavirus and identify factors that influence how the virus is transmitted.
This includes how a person who is infected with Covid-19 virus transmits infectious virus particles into the environment.
The human challenge study is being delivered by a partnership between the UK government’s Vaccines Taskforce, Imperial College London, the Royal Free London NHS Foundation Trust and hVIVO.
The Royal Free Hospital’s specialist and secure clinical research facilities in London are specifically designed to contain the virus.
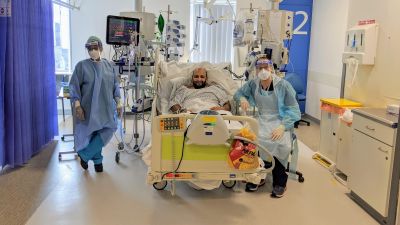
Highly trained medics and scientists will be on hand to carefully examine how the virus behaves in the body and to ensure the safety of volunteers.
The virus being used in the characterisation study has been produced by a team at Great Ormond Street Hospital for Children NHS Foundation Trust in London, in collaboration with hVIVO with support from virologists at Imperial College London.
If you live in Northern Ireland or Britain you can express an interest in taking part in this research at ukcovidchallenge.com







